PATENT
A patent was filed by the inventors to treat and eliminate Grover’s Disease, GD (transient acantholytic dermatosis -TAD, persistent acantholytic dermatosis and transient acantholytic dyskeratosis).
The treatment was applied by topical administration of a propietary gel formulation , comprising active ingredients that are GRAS (Generally Recognized As Safe). The active ingredients are considered biologically active and safe.
APPLICATION:
The application included the administration of the proprietary gel every morning for 30 days, after shower, to the clean, thoroughly dried surface area.
In the evening, before bedtime the proprietary gel was also applied without any preparation of the affected area for 7 days.
1-day
Before Treatment, Raw painful blisters

1-7 days:
As described above, the propietary gel was applied twice a day and our patients said that after 2 days they were able to feel for the first time a sense of relief from discomfort of irritation since their GD symptoms appeared.
Crusting started after 5-6days
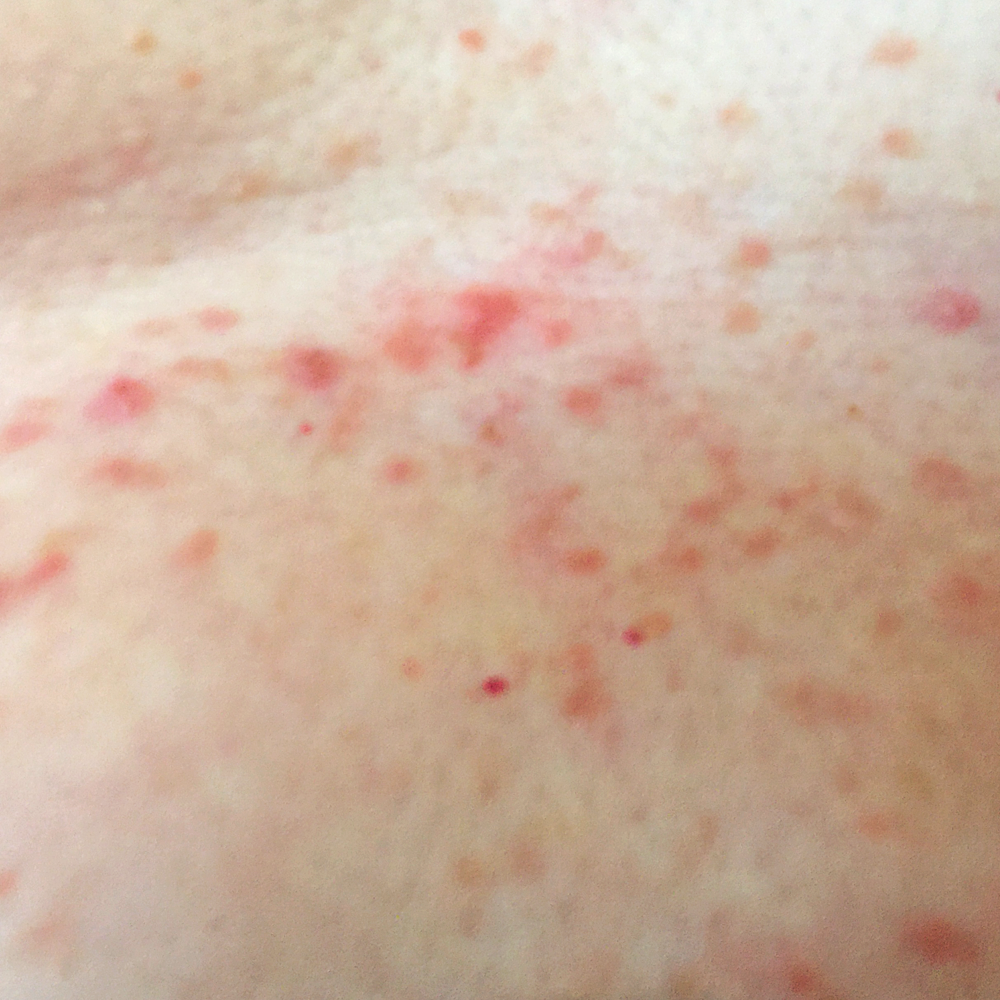
After Day 7:
Once a day application
Only the morning routine was followed.
Discomfort significantly diminished.
After 2 weeks :
The red, inflamed outbreaks, started to heal and turned brownish. The heights of the blisters started to diminish. Contraction and epithelialization occurred.
Healing

DAY 21 :
The only remains of GD were the light brown spots on the skin where the blisters used to be.
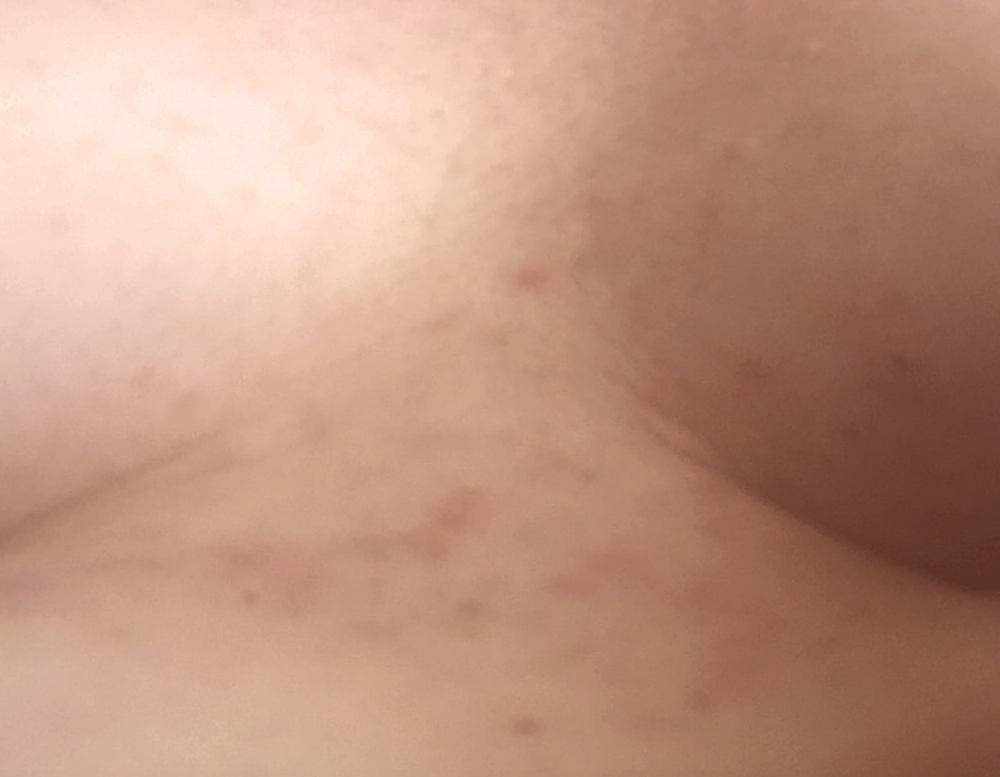
fading scars
DAY 30 :
The gel application were discontinued. Discomfort significantly diminished
.
After 3 months:
No reoccurrence of GD. The light brown spots (as mentioned above under day21) were slowly fading.
After 6 months
In the wintertime: no outbreak but a sensation of slight discomfort was reported on the skin where the GD used to be, but after one application of the gel it disappeared.
